Material Design: Probes and Labels for Biomedical Applications
Novel advanced red and near-infrared (NIR), extremely bright and photostable probes and labels for biomedical applications (Seta and Square series), fluorescence lifetime (FLT) markers (Seta and SeTau series), real dark quenchers (SQ series), and fluorescent classification (coding) dyes were developed and commercialized. These materials are useful for biomedical and pharmaceutical assays based on fluorescence intensity, polarization, lifetime, or fluorescence resonance energy transfer (FRET). These materials, which have certain advantages over dyes of Cy, Alexa, DyLight, and ATTO series, include:
- Amine- and thiol-reactive red and NIR fluorescent labels and click-chemistry reagents of the Square and Seta series for covalent attachment to biomolecules, such as proteins, amino acids, peptides, oligonucleotides, DNA, RNA, lipids, and drugs;
- Fluorescent probes for noncovalent labeling of proteins, lipids, and cells;
- Fluorescent dyes for two-photon excitation;
- Highly bright and highly photostable fluorescent probes and labels for fluorescence imaging and high resolution imaging applications;
- Fluorescent dyes and tandems with fluorescent proteins for flow cytomentry which are compatible with 405, 488, and 633-nm light sources;
- pH-sensitive probes and labels;
- Fluorescence lifetime (FLT) probes and labels of SeTau series for FLIM, polarization and FLT-based applications;
- Donor-acceptor pairs and reactive, dark quenchers (SQ) for fluorescence resonance energy transfer (FRET) applications;
- Highly stable and low blinking, long-wavelength fluorescent dyes for single molecule applications;
- Fluorescent classification (coding) dyes for single or multiple encoding of microspheres used in high-throughput screening (HTS) and multiplexed analysis systems (MAS).
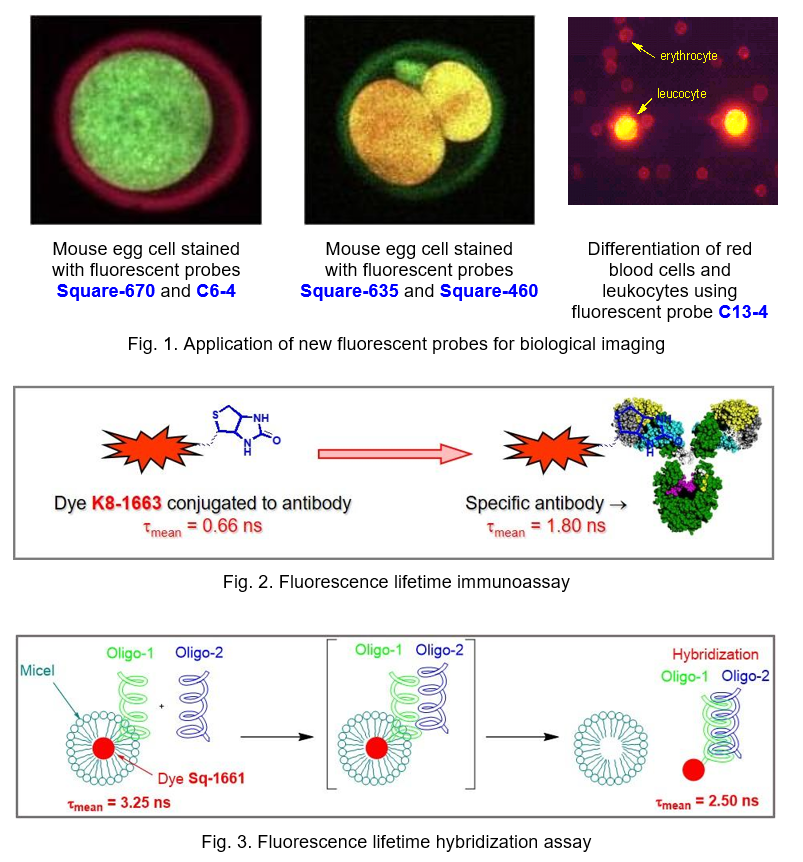
Environment sensitive fluorescent dyes. Based on styryls, squaraines, benzanthrones, and naphthalic acid derivative a series of new fluorescent dyes that are sensitive to the microenvironment, including pH, hydrophobicity, polarity, and the presence of proteins were developed. These dyes are promising fluorescent bioanalytical reagents, probes and labels with improved characteristics for clinical diagnostics, biomedical and pharmaceutical assays. These new materials are used to study the intracellular pH, albumin, red blood cells, and cell membranes, including fluorescence imaging applications (Fig. 1). The fluorescence lifetime immunoassay (Fig. 2) and hybridization assay (Fig. 3) were also developed based on these dyes.
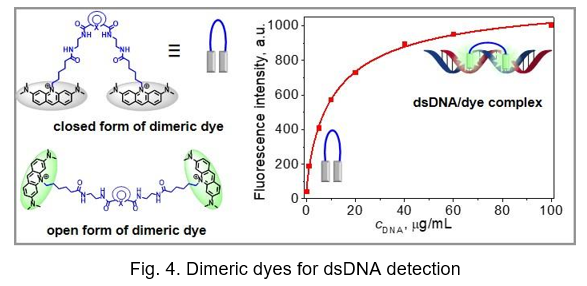
Probes for nucleic acids detection and PCR analysis. Novel homodimeric dyes based on the acridine orange (AO) chromophore (Fig. 4) were recently synthesized. Their photophysical properties measured free in solution and on binding to double-stranded DNA (dsDNA) were compared to those of widely used nucleic acid labelling dyes, namely SYBR Green I (SG) and YOYO-1. Contrary to SG and YOYO-1, the obtained dyes showed better resistance to high temperatures, light and oxidizing reagents. The inhibitory effect and the effect on DNA melting temperature in a real-time quantitative polymerase chain reaction (qPCR) for dimeric dyes were less inhibitory to qPCR than SG at a broader range of dye concentrations (i.e. 0.75–1.75 μM).
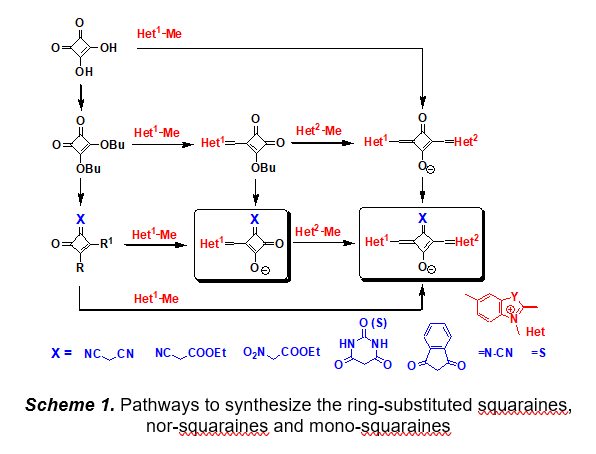
Reactions of squaric acid, dibutyl squarate and mono-squaraines with CH-acidic compounds, nitrogen containing heterocyclic methylene bases, cyanamide and thionating reagents have been investigated (Scheme 1). As a result, preparative methods for the synthesis of practically important squaraines and nor-squaraines were developed and the novel highly bright and photostable fluorescent dyes were synthesized. Correlations between spectral, photophysical and photochemical properties and the molecular structure of these dyes were obtained.
Novel water-soluble cyanine and squaraine dyes. A series of monoreactive, water-soluble, squaraine dyes with two aromatic sulfo groups and up to 3 sulfobutyl groups was synthesized and the spectral, photophysical and photochemical properties of these dyes were compared to dicarbocyanines of the same structure. Compared to cyanines the squaraine dyes absorb and emit in aqueous solutions at shorter wavelengths (630–636 nm / 639–645 nm vs. 647–653 nm / 665–672 nm), have higher molar absorptivities (284,000–333,000 M–1cm–1 vs. 242,000-260,000 M–1cm–1), lower fluorescence quantum yields (4.3–9.4% vs. 27–32%), and lower fluorescence lifetimes (0.2–0.3 ns vs. 1.0–1.2 ns) but the quantum yields and lifetimes substantially increase when bound to protein (BSA) or antibody (IgG). Compared to cyanines, squaraine dyes exhibit higher photostabilities and much higher sensitivity of the quantum yields and fluorescence lifetimes towards the microenvironment and are therefore better suited as biomedical fluorescence sensors.
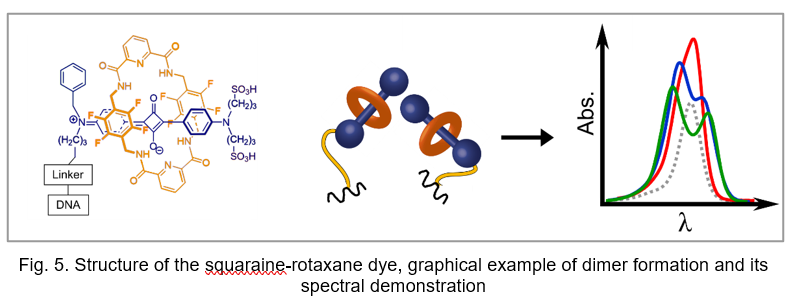
Extremely bright, chemically and photochemicalyl stable fluorescent dyes, probes and labels based on nano-encapsulated squaraines. The formation of a squaraine-rotaxane (SR) system results in an increase in quantum yields, fluorescence lifetimes, photo- and chemical stability. The dramatic changes in spectral and photo-physical characteristics are due to 3D sterical protection of central squaraine fluorophore system against micro-environmental impacts. Moreover, squaraine-rotaxanes can form very rare oblique packing, which may benefit applications involving energy transfer, light harvesting, and quantum information. This is confirmed by the presence of excitonically split bands with almost equal transition intensities (Fig. 5) after templating of two squaraine rotaxanes dyes on DNA, which is typical just for oblique packing.
Sensing the Microenvironment. Red and NIR probes and labels of Seta and especially Square series show noticeable increases of fluorescence intensity and longer fluorescence lifetimes in presence of large biomolecules such as proteins and lipids. Fluorescence intensity increases of up to 100 times and higher were observed. The fluorescence intensity of dyes such as K8-1355, K8-1431, K8-1440, and K8-1500 substantially increases upon binding to BSA or other large biomolecules. The quantum yields for some of the non-covalent complexes of these dyes with BSA are as high as 80%. Therefore these probes are perfectly suited for fluorescence-based quantification of proteins. Because these dyes readily stain cells of different nature they are also useful for in-situ biological imaging. Furthermore they are useful as stains for gel electrophoresis. Due to the fact that the protein binding constants of these dyes are comparable to those of some common drugs, they are used for the assessment of drug-binding constants by the dye-displacement method.