Substance structure and composition: smart and valid
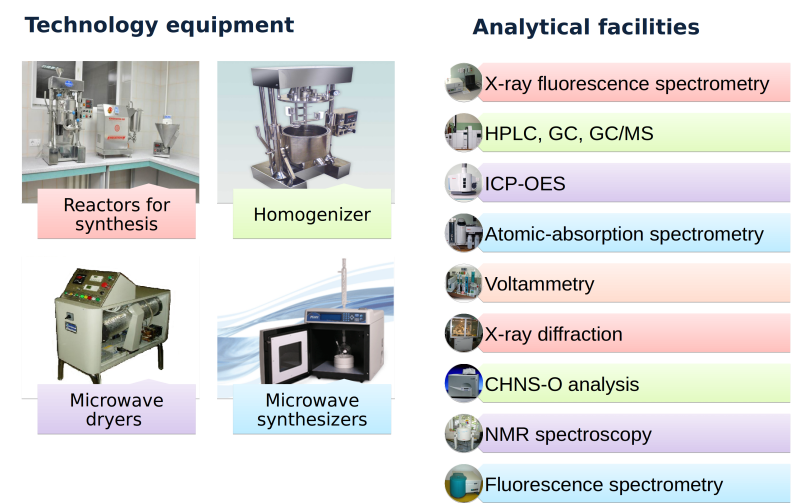
NMR spectroscopy
X-ray diffraction
XRD of organic compounds. XRD of powders including crystal structure
studies using Rithweld’s method.
HPLC, GS/MS
Determination of organic compounds in liquid and solid samples (NIST 02
base with data of approx. 175 000 compounds is available).
ICP AES, AES and AAS
Analysis of extra pure salts. Analysis of alloys containing precious metals.
Analysis of natural and drinking water for contamination of 33 elements
and major anions. Soil and air analysis for main components and trace-
level impuritites.
Elemental analysis
CHNS-O analysis of organic compounds.
X-ray fluorescence
WD-XRF and GE-XRF of alloys and solid samples.
Fluorescence lifetime and steady-state spectroscopy
UV-vis spectroscopy
Synthesis of organic and inorganic compounds:
Synthesis of organic compounds including dyes and luminophors, biologically active compounds, component of liquid crystals, etc. Custom synthesis of compounds, combinatorial libraries and building-blocks. Development of synthetic approaches and schemes. optimization of organic and inorganic synthesis. Microwave- and ultrasonic-assisted synthesis.
Microwave equipment:
Design of unique laboratory and industrial microwave equipment for drying, sterilization, evaporation, distillation and other purposes by request. Production of designed microwave equipment. Development of technologies based on microwave equipment. Optimization of industrial technologies with application of microwave equipment.
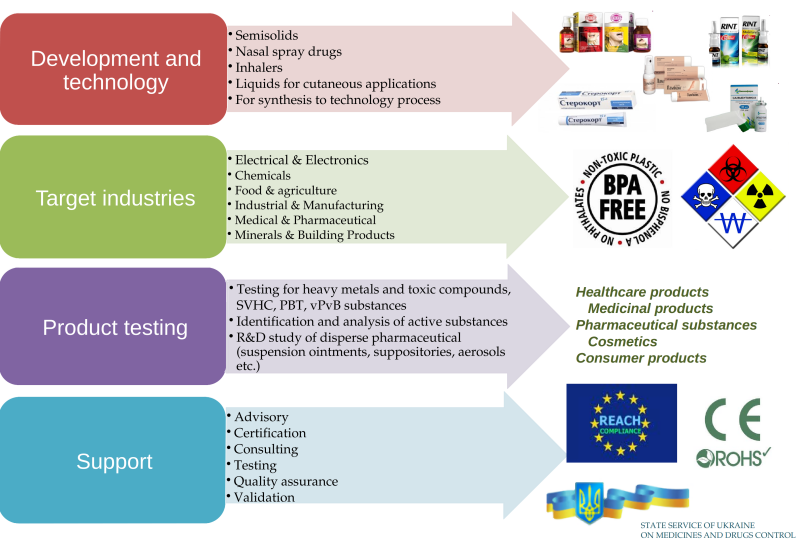
Research and development of medicinal products:
Development and research of medicinal products: topical semi-solid preparations (ointments, creams, gels, pastes); liquid preparations for cutaneous application – lotions, shampoos etc.; oral liquids – solutions, emulsions and suspensions, syrups); nasal preparations (drops and sprays); preparations for inhalation (pressurized metered-dose inhalers); suppositories (rectal, vaginal).
Development of analytical procedures for quality control of medicines, validation of analytical procedures.
Stability testing of medicinal products.
Regulatory services:
Preparation of registration documentation for medicinal products.
Drafting of scientific guidelines and regulatory documents for pharmaceutical industry.