Liquid crystal materials
Design and studying of high-performance ferroelectric liquid crystal (FLC) materials targeted on high demand applications in display and photonic devices is described. Combination of high twisting ability with large spontaneous polarization (PS>100 nC/cm2) resulted in promising FLC mixtures: almost defect-free alignment in electro-optical cells, the optical quality of the cells becomes comparable to that based on nematic liquid crystals, but switching is at least in order faster. The most important parameter is responsible for the remarkable erformance of the materials is their ultra-short helix pitch as low as 65 nm. The key components of new mixtures provided the advanced properties are high-twisting diesters of terphenyldicarboxylic acid and chiral 1,1,1-trifluoroalkan-2-ols (FOTDA-n, n = 4–8). Analysis of origin of twisting power in SmC* phase, main difference with N* LC, relationship with chemical structure of chiral components are given. Thus, remarkable increase (up to 2.8-fold) of helical twisting power in the smectic C* (SmC*) phase upon lengthening of terminal alkyl chains from 4th to 8th homologues is observed for FOTDA-n. New chiral compounds, FOTDA-n, where n is 5 and higher at concentrations as low as 25–33 mol.% in non-chiral BPP based SmC* host induce the helix pitch considerably below 100 nm.

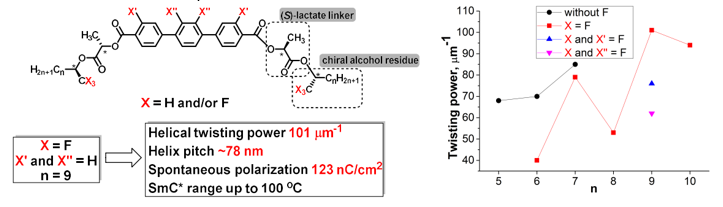
Design and studying of derivatives of fluorinated and non-fluorinated p-terphenyl-containing symmetric tetraesters as chiral components (CCs) of ferroelectric liquid crystal (FLC) materials working in deformed helix FLC (DHFLC) mode are described. The tetraesters combine a lactate and alcoholic residues in chiral tails. The new CCs induce short helix pitch (as short as 78 nm) and large spontaneous polarization (PS>100 nC/cm2) at moderate concentrations (as low as 12 mol. %) in a non-chiral biphenylpyrimidine host. We have studied the effects of the (S)-lactate linker, terminal alkyl chains and fluorine atoms on helix pitch and other useful properties of the chiral tilted smectic (SmC*) phase induced by these CCs in the biphenylpyrimidine host.
Depending on positions of the fluorine atoms, the fluorination of the p-terphenyl core shows little effect or even reduces the helical twisting power (HTP) of the CCs. At the same time, modifications in the chiral tails can essentially enhance the HTP and other CC performance characteristics. Thus, the combination of the (S)-lactate linker with a (S)-alkan-2-olic residue results in high HTP (up to 85 µm–1) even without fluorine atoms in the chiral tails. Substitution of the (S)-alkan-2-olic residue with a fluorinated analogue, viz. (R)-1,1,1-trifluoroalkan-2-ol derivative, provides further increase of the HTP in the induced SmC* phase up to the highest known to date value of 101 µm–1.
Organic synthesis
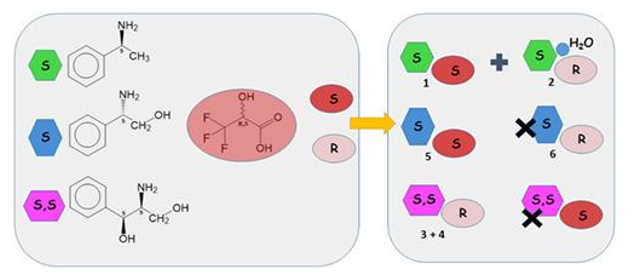
Resolution of rac-3,3,3-trifluorolactic acid by diastereomeric salt formation was reinvestigated. The use of (S)-1-phenylethylamine gives coprecipitation of two diastereomeric phases, 1 (S)-[NH3CH(CH3)Ph](S)-[CF3CH(OH)COO] and 2 (S)-[NH3CH(CH3)Ph](R)-[CF3CH(OH)COO]·H2O.
Pure phase 1 may be obtained using molecular sieves as desiccants. Resolution by (S,S)-2-amino-1-phenylpropan-1,3-diol gives monoclinic (S,S)-[NH3CH(CH2OH)CHOHPh] (R)-[CF3CH(OH)-COO] 3 with minor (S)-3,3,3-trifluorolactate contamination, which is precluded in the recrystallized orthorhombic form 4. A new resolution using inexpensive phenylglycinol gives pure phase 5 (S)-[NH3CH(CH2OH)Ph] (S)-[CF3CH(OH)COO] in 76% yield, 94% ee in a single step, in preference to its (S)-(R) diastereomer 6. Overall efficient resolution for both enantiomers of the trifluorolactic acid (each ca. 70% yield, 99% ee) may be achieved by various two-step “tandem” crystallizations, involving direct addition of either water or a second base to the filtrate from the initial reaction.
The LDA-mediated reaction of 4-methyl-1-phenylquinolone and 3-methyl-2-(methylthio)benzothiazolium tosylate proceeds differently depending on the amount of LDA used. With about 1 eq. LDA, the known condensation reaction takes place, and the expected benzothiazolic derivative forms. However, when 2 eq. LDA or more is used, a new reaction occurs by an interaction of the condensation product with an excess of LDA and results in cleavage of the C–S bond in the thiazole moiety along with formation of the other C–S bond in the quinolone moiety to give the 2-aminothienoquinolone derivative.;