With application of convenient thermal heating and non-classical activation methods – microwave- and ultrasonic-assisted synthesis - the strategy of selectivity tuning some multicomponent condensations for synthesis of diverse types of heterocyclic compounds has been developed.
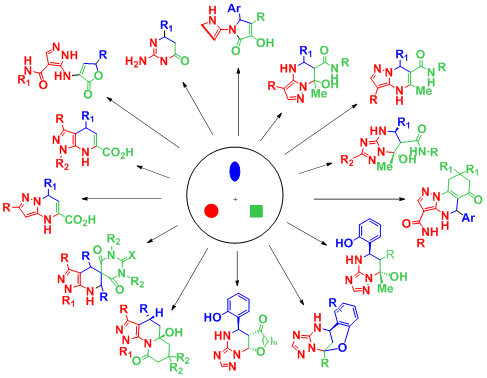
There were discovered and studied the new multicomponent and modified reactions leading to potentially pharmacologically active heterocycles, including analogues of some natural compounds and known drugs.
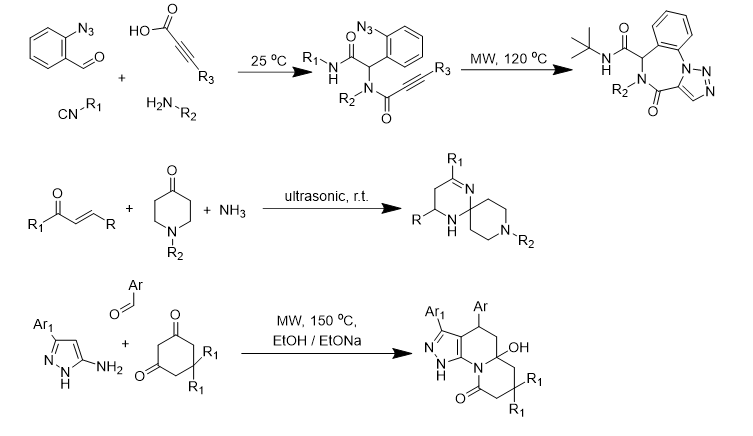
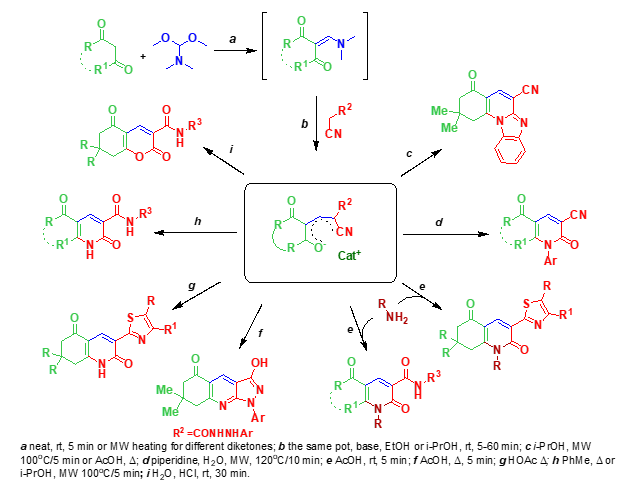
Multidirectional reactions of resonance-stabilized enolates were studied and depending of the treatment direction on the conditions was shown. Several possible courses of the reactions were investigated and optimal parameters of the processes for selective formation of the certain heterocycles were established.
Searching substances for selective inhibition of enzyme 11β-HSD1 (11β-Hydroxy Steroid Dehydrogenase type 1) – a drug-target for treatment of disorders with cortisol excess such as obesity, insulin resistance and type 2 diabetes mellitus, and SIRT1 (NAD-dependent deacetylase sirtuin-1) activators – potential agents for age dependent metabolic abnormalities treatment (in cooperation with other departments scientists and specialists of SI “V. Danilevsky Institute for Endocrine Pathology Problems of the National Academy of Medical Sciences of Ukraine”, Kharkiv).
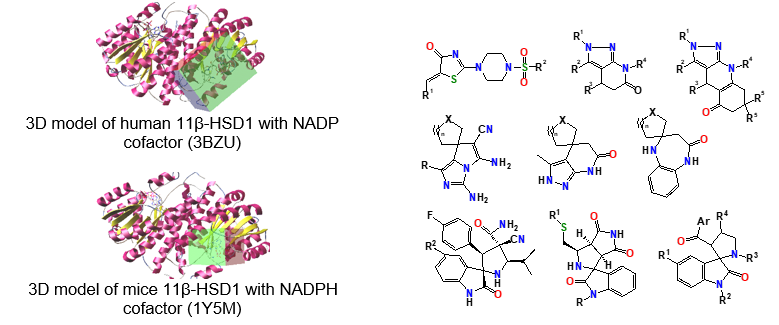
Synthesis and molecular structure investigation of fused and spiro-organized nitrogen containing heterocycles for different pharmacological purposes.
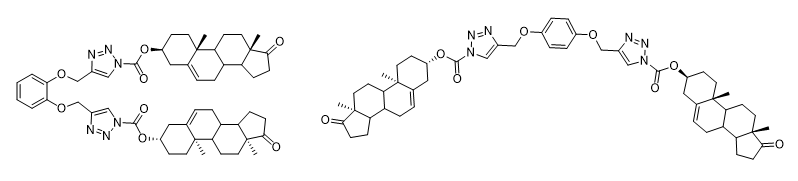
New isomeric supramolecular dehydroepiandrosterone appended 1,2,3-triazole-based potential gelators were synthesized by the click chemistry method and their ability to form gels in different solvents was studied experimentally as well as at the molecular level using molecular dynamics and quantum chemistry. It was proposed two modes of gelation of the compounds resulting from the competition of molecular entanglement and binding site interactions, which strongly depend on the molecular spacer structure.
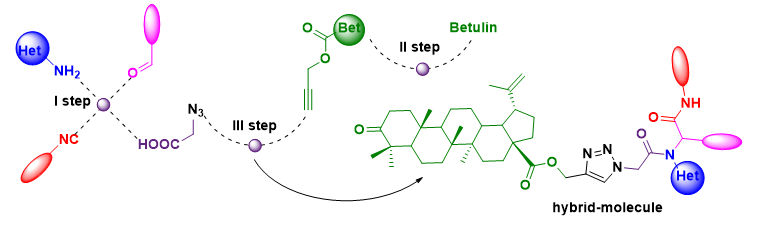
A combination of the Ugi reaction with the azide-alkyne cycloaddition reaction was used to modify betulin by step-by-step transformation.
New agrochemical formulations for post-harvest treatment of fruits based on supramolecular complexes of 1-methylcyclopropene (1-MCP) with nanocarriers (cucurbit[6]uril, α-cyclodextrin) and various additives were developed, aimed at prolongations of fruits shelf life. These preparations produced on a commercial scale, after undergoing successful laboratory and field trials, have been introduced into the Ukrainian agricultural market.
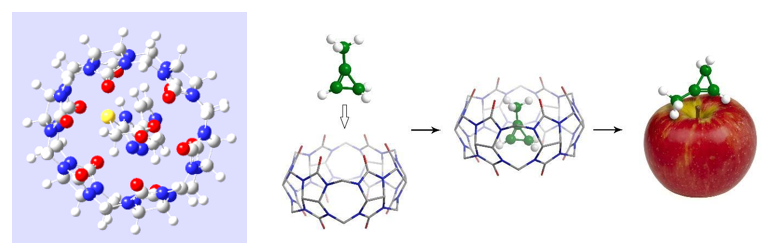
For controlling the content of 1-MCP in the air of fruit storages during apples treatment, the new methodology for assaying 1-MCP in gas-air mixtures after decomposition of complexes and the release of 1-MCP from preparations was proposed.
A convenient method of chemical modification of 1-methylcyclopropene was developed to convert it to a more stable substance - dimethyl 2-(2-methylcycloprop-2en-1-yl) maleate by reaction with dimethyl acetylenedicarboxylate. A method for quantitative analysis of the obtained derivative and for analysis of 1-methylcyclopropene in the complex by extraction in solution, derivatization and analysis of the derivative by high performance liquid chromatography was elaborated.
Presently, the research studies of new supramolecular complexes of cucurbiturils [6,7] with various 1,2,4-triazolylthioacetic acid derivatives, the latter applied in agriculture as fertilizers or as veterinary drugs, are being carried out. These complexes can have potential for the development of new preparations with improved properties, providing more convenient and safe use of the drugs, e.g. prolonged release of the active substance from the preparations.